Device Identification

Acuitytec Device Authentication Fingerprinting And Id Fraud Prevention

Udi Unique Device Identification Fda Udi Infofda Udi Info

What Is Udi Unique Device Identification Tsquality Ch

Unique Device Identification Udi

Unique Device Identification System Udi System Fda
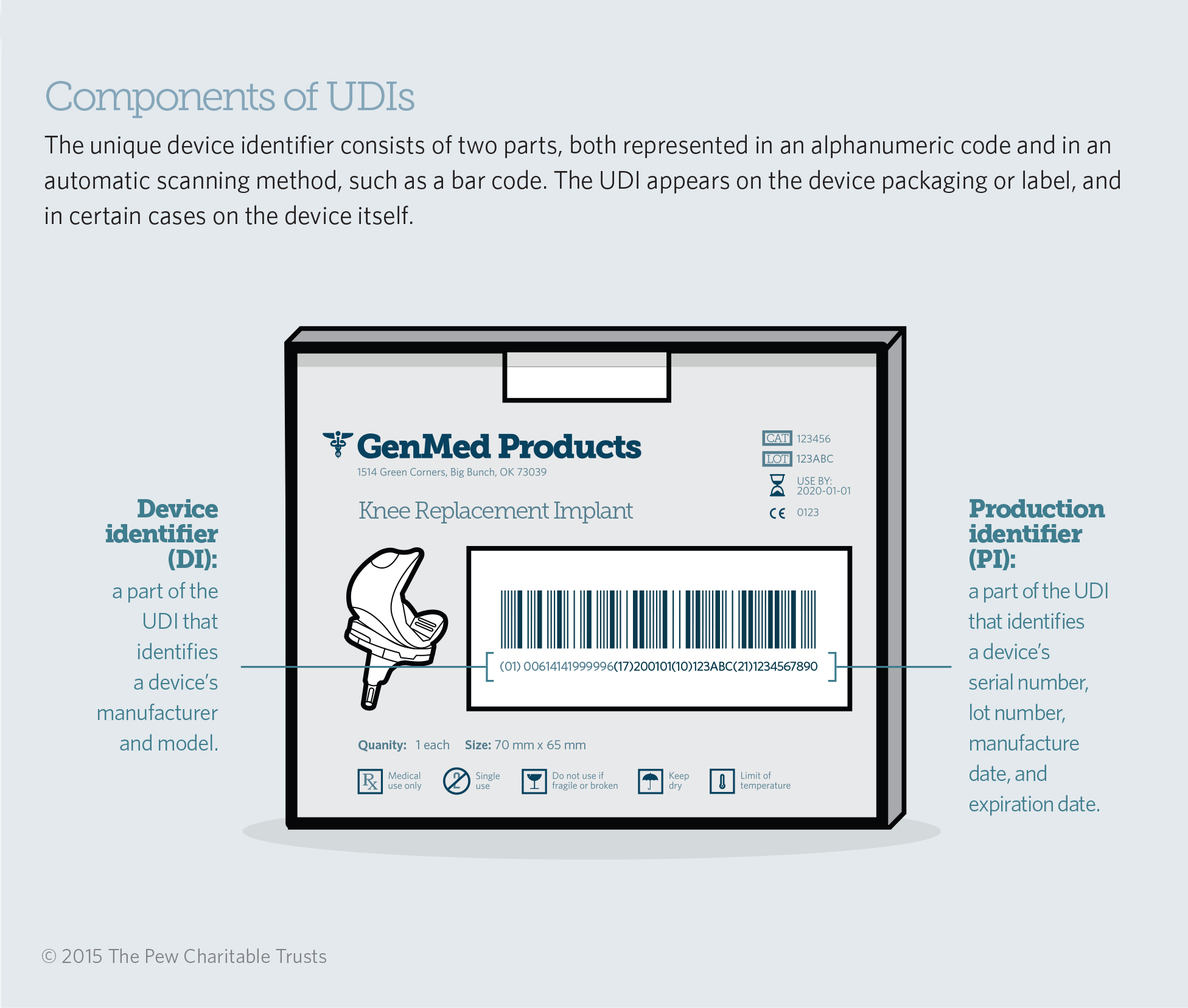
Implementing Unique Device Identification The Pew Charitable Trusts
DICE Device Identifier Composition Engine Introduction DICE (previously called RIoT) is a family of hardware and software techniques for hardwarebased DICE in a Nutshell Many SOCs contain fusebanks (or other NVmemory) that can be used to store cryptographic keys for Balancing Cost,.

Device identification. The two new EU Regulations on medical devices 745/17 (MDR) and 746/17 (IVDR) introduce the Unique Device Identifier System (UDI)Those codes are made of a series of alphanumeric characters that will allow to univocally identify any medical device, except for custommade and performance study/investigational devices The UDI system will facilitate easier traceability of medical devices. A device has only one device ID A device ID has the same format as a hardware ID The Plug and Play (PnP) manager uses the device ID to create a subkey for a device under the registry key for the device's enumerator To obtain a device ID, use an IRP_MN_QUERY_ID request and set the ParametersQueryIdIdType field to BusQueryDeviceID. Devicefree passive identity identification attracts much attention in recent years, and it is a representative application in sensorless sensing It can be used in many applications such as intrusion detection and smart building Previous studies show the sensing potential of WiFi signals in a devicefree passive manner.
The official name of the identification device is Stealth and the innovative gadget works to verify one's identity through mouth biometrics The minds behind this new way of storing and accessing sensitive digital information are Beren Kayali, Lu Ye, Paul Mendieta, and Lea Marolt Sonnenschein. A UDID is an abbreviation utilized for Unique Device ID It is an element of Apple iPhones and their different gadgets It is a 40 digit series of numbers, that is useful in representing your device Every individual Apple gadget has its ID that is being gotten by Apple servers When testing another versatile application on an Apple gadget we. The Unique Device Identification (UDI) System is intended to assign a unique identifier to medical devices within the United States, Europe, China, South Korea, Saudi Arabia and Taiwan It was signed into law in the US on September 27, 07, as part of the Food and Drug Administration Amendments Act of 07The EU acted to adopt UDI and on April 5, 17, under the EU Medical Device Regulation.
Device ID Have an unknown device?. Find your Amazon Device ID 1 Start at the Tubi home screen, then navigate to the left until you see a list of menu options Click on the Settings 2 In settings, navigate to the About icon, and underneath "Need Help?" you will find your device ID 3 Snap a picture of the abovementioned screen. Device Description The ID NOW™ Instrument is a portable, fluorescence based instrument intended to be used for the processing and analysis of Abbott molecular diagnostic assays.
The Global Unique Device Identification Database (GUDID) is a database administered by the FDA that will serve as a reference catalog for every device with a unique device identifier (UDI) GUDID. A Unique Device Identification (UDI) system is intended to provide single, globally harmonized positive identification of medical devices through distribution and use, requiring the label of devices to bear a globally unique device identifier (to be conveyed by using Automatic Identification and Data Capture and, if applicable, its Human Readable Interpretation) based upon standard, with the UDIDI (Device Identifier) of that unique identifier being also linked to a jurisdictionspecific. The two new EU Regulations on medical devices 745/17 (MDR) and 746/17 (IVDR) introduce the Unique Device Identifier System (UDI)Those codes are made of a series of alphanumeric characters that will allow to univocally identify any medical device, except for custommade and performance study/investigational devices The UDI system will facilitate easier traceability of medical devices.
Scroll down to the Devices section To see the serial and IMEI/MEID number, select the device If you still need help, here's what to try next Do you have a different device with iOS 103 or later that's signed in to your Apple ID?. On that device, go to Settings > Your name Scroll down to see any devices signed in with your Apple ID. A Device ID is a string of numbers and letters that identifies every individual smartphone or tablet in the world It is stored on the mobile device and can be retrieved by any app that is downloaded and installed Apps typically retrieve the ID for identification when talking to servers.
A device ID (device identification) is a distinctive number associated with a smartphone or similar handheld device Device IDs are among one of the easiest ways to identify mobile users Device IDs are among one of the easiest ways to identify mobile users. A Unique Device Identification (UDI) system is intended to provide single, globally harmonized positive identification of medical devices through distribution and use, requiring the label of devices to bear a globally unique device identifier (to be conveyed by using Automatic Identification and Data Capture and, if applicable, its Human. The first step is to see whether the patient has a device identification card or knows the device manufacturer If such information is not available, then access or obtain a chest Xray (step 2) Step 3 involves identifying the ra diopaque manufacturerspecific ANC;.
Device Identification is an excellent subsequent visit authentication mechanism, to be able to say the user in a subsequent visit is using the same computer as the last time they came to the site Additionally, it provides strong tools for linking mulitple accounts to the same device Device Identification works well for digital products, where. Regulatory agencies increasingly require a unique device identifier (UDI) to ensure a medical device has future traceability to a manufacturer The objective is to unambiguously identify all medical devices in the healthcare supply chain through distribution and use. Get To Know Your Users In today’s mobile ecosystem, smartphones are vital for their users, almost like the air they Associate Each Device To A Specific User While a computer can be used by many users, a mobile device usually.
Device identification To identify a device with Windows Autopilot, the device's unique hardware hash must be captured and uploaded to the service This step is ideally done by the hardware vendor (OEM, reseller, or distributor) automatically associating the device with an organization. Devicefree passive identity identification attracts much attention in recent years, and it is a representative application in sensorless sensing It can be used in many applications such as intrusion detection and smart building Previous studies show the sensing potential of WiFi signals in a devicefree passive manner. UNIQUE DEVICE IDENTIFICATION HEALTHCARE PROFESSIONALS OVERVIEW Regulatory agencies increasingly require a unique device identifier (UDI) to ensure a medical device has UNDERSTANDING UDI AND GS1 Medtronic manages all global regulation for standard product information by following GS1 Unique.
Medical device manufacturers registered with FDA and medical devices listed with FDA Note Registration of a device establishment, assignment of a registration number, or listing of a medical device does not in any way denote approval of the establishment or its products by FDA. When fully implemented, the Unique Device Identification System will offer a range of benefits to industry, FDA, consumers, health care providers and health care systems by Allowing more accurate. Identification number or through a biometric identification device, for example through thumb print recognition.
Network Device Identification (NDI) uses broadcast messaging data to help train the machine learning algorithms of the Sense home energy monitor Here at Sense, we’re hungry for the best data we can get about your devices. Note Registration of a device establishment, assignment of a registration number, or listing of a medical device does not in any way denote approval of the establishment or its products by FDA. Unknown Device Identifier enables you to identify the yellow question mark labeled Unknown Devices in Device Manager and reports you a detailed summary for the manufacturer name, OEM name, device type, device model and even the exact name of the unknown devices.
Organizational devices requiring unique devicetodevice identification and authentication may be defined by type, by device, or by a combination of type/device Information systems typically use either shared known information (eg, Media Access Control MAC or Transmission Control Protocol/Internet Protocol TCP/IP addresses) for device identification or organizational authentication solutions (eg, IEEE 8021x and Extensible Authentication Protocol EAP, Radius server with EAP. The two new EU Regulations on medical devices 745/17 (MDR) and 746/17 (IVDR) introduce the Unique Device Identifier System (UDI)Those codes are made of a series of alphanumeric characters that will allow to univocally identify any medical device, except for custommade and performance study/investigational devices The UDI system will facilitate easier traceability of medical devices. On iOS, a device ID is called the ‘Identity For Advertisers’ ( IDFA, or IFA for short) On Android, the device ID is the GPS ADID (or Google Play Services ID for Android) A user is able to access their GPS.
The way you make it discoverable depends on the device Check the device or visit the manufacturer's website to learn how On your PC, select Start > Settings > Devices > Bluetooth & other devices > Add Bluetooth or other device > Bluetooth Choose the device and follow additional instructions if they appear, then select Done. According to Gartner, every marketer should have crossdevice identification (XDID) technologies on their radar The research firm highlighted XDID as one of six marketing technologies to watch. If you have reinstalled windows or plugged in a device that isn't working this tutorial will help you find device information and drivers How to identify an unknown device Common PCI Vendors AMD/ATI Vendor Information & Devices Intel.
Medical devices listed with FDA;. The device ID of your Android tablet can be helpful if you have problems with your phone You will need to contact customer service to resolve the problem when asked for device ID details Method 2 Use Android device ID changer app to change device ID If you have a rooted device, then changing device ID is just a twotap process. The two new EU Regulations on medical devices 745/17 (MDR) and 746/17 (IVDR) introduce the Unique Device Identifier System (UDI)Those codes are made of a series of alphanumeric characters that will allow to univocally identify any medical device, except for custommade and performance study/investigational devices The UDI system will facilitate easier traceability of medical devices.
Tips for better search results Ensure correct spelling and spacing Examples "paper jam" Use product model name Examples laserjet pro p1102, DeskJet 2130 For HP products a product number Examples LG534UA For Samsung Print products, enter the M/C or Model Code found on the product labelExamples. For all USB devices, the USB bus driver generates a standard set of identifiers composed of values retrieved from the USB device and interface descriptors Standard USB Identifiers are discussed in the first of the two sections indicated above In addition to the standard USB identifiers, native Windows drivers for mass storage and printer. The Global Unique Device Identification Database (GUDID) contains key device identification information submitted to the FDA about medical devices that have Unique Device Identifiers (UDI) The FDA is establishing the unique device identification system to adequately identify devices sold in the US from manufacturing through distribution to patient use.
Deploy DeviceID on firewalls that are centrally located in your network For example, if you have a large environment, deploy DeviceID on a firewall that is upstream from the IP address management (IPAM) device If you have a small environment, deploy DeviceID on a firewall that is acting as a DHCP server. How To Track Device ID And How To Use Device IDs For Mobile Marketing?. With this simple app, you can easily get, copy and share Device ID (Android ID) IMEI SIM serial, Android version and many more Totally SAFE and FREE If you like this app, please support us by giving it a good rating.
AccessGUDID is a searchable database of device identification information, such as the device identifier on the label, device name, company name, MR safety status, and premarket submission numbers. Device identification and authentication technology trends and strategies bank information security Connected IoT devices are expected to reach more than 75 billion by 25 Because of that. If not identifiable, then proceed to step 4.
METHOD 1 Identification using ODIS via AT&T LwM2M Client Your chosen radio module within the device must support AT&T's LwM2M client If it does not then this method is not available to you However, you can still certify your device with AT&T using either Method 2 or 3 below Click here to learn more. Adversaries may perform control device identification to determine the make and model of a target device Management software and device APIs may be utilized by the adversary to gain this information By identifying and obtaining device specifics, the adversary may be able to determine device vulnerabilities This device information can also be used to understand device functionality and inform the decision to target the environment. Huntersoft's Unknown Device Identifier is a free tool that scans your PC to identify any devices that Windows doesn't recognize It can also find and install drivers for devices.
Here are two different steps to Change the device id and device info and identity of any android device First is to use Device ID Changer android app to change device ID of any android phone and second is to change device Info and Identity of any android device with Xposed module that will let you change your device identity to fake it for certain apps in order to perform lots of tweaks. Device Identification, Registration and Blocking System’s (DIRBS) is a mobile device ecosystem implemented by PTA to ensure that only type approved and legal devices are operational over mobile networks in Pakistan How to check IMEI?. If you have reinstalled windows or plugged in a device that isn't working this tutorial will help you find device information and drivers How to identify an unknown device Common PCI Vendors AMD/ATI Vendor Information & Devices Intel.
You can use the Vendor and Device IDs you extracted above to search the database at devicehuntcom Enter the fourdigit Vendor ID (VEN_XXXX) into the Vendor ID search field, or the fourdigit Device ID (DEV_XXXX) into the appropriate field and click the "Search" button The database is extensive but does not contain every piece of hardware. This database includes medical device manufacturers registered with FDA and;. Device identification and authentication technology trends and strategies bank information security Connected IoT devices are expected to reach more than 75 billion by 25 Because of that.
CDI is the Compound Device Identity A value that depends on both the hardware and the software that booted UDS is the latchable Unique Device Secret, held in fuses or other readonly storage HMAC is a keyed hash function Hash(program) is the cryptographic hash of the code that starts executing at powerup or reset. POST /enterprises/projectid/devices/deviceidexecuteCommand { "command" "sdmdevicescommandsThermostatEcoSetMode", "params" { "mode" "MANUAL_ECO" } } Response This command impacts other. Unique Device Identifier On September 24, 13 the US FDA released a final rule requiring that medical devices distributed in the US carry a Unique Device Identifier (UDI) and that product information pertaining to the devices be submitted to the FDA's Global Device Identification Database (GUDID).
Kindly dial *#06# from the dialer of mobile device to know your 15 digit IMEI number. DeviceID requires an IoT Security license, a Cortex Data Lake (CDL) license, and the device certificate If you use PANOS version 810 through PANOS 91x on a firewall, the IoT Security license provides device classification, behavior analysis, and threat analysis for your devices. The two new EU Regulations on medical devices 745/17 (MDR) and 746/17 (IVDR) introduce the Unique Device Identifier System (UDI)Those codes are made of a series of alphanumeric characters that will allow to univocally identify any medical device, except for custommade and performance study/investigational devices The UDI system will facilitate easier traceability of medical devices.
Manufacturers are required under the UDI rule to mark Class I, Class II, and Class III medical device packages with a unique device identifier and standardized date and to upload the device data to the FDA’s Global Unique Device Identification Database (GUDID) Implementation is well underway for Class II, Implantable, Life Sustaining/Life Supporting, and Class III devices (compliance deadlines have already past). In settings, navigate to the About icon, and underneath "Need Help?" you will find your device ID 3 Snap a picture of the abovementioned screen and send it over to support@tubitv, along with a short description of the problem you may be experiencing and we'll assist you ASAP!. Device ID Have an unknown device?.

Unique Device Identification For Medical Devices Rea Jet

U S Fda S Unique Device Identifier Udi Requirements Youtube
Q Tbn And9gcsewcyvn6 Upfmtbvfnfv0vyyzb7vkpgxucsyrwyarmamfvcahw Usqp Cau

Beckhoff Information System English

Device Identification Icons Missing Support Roon Labs Community

What Is The Unique Device Identification Udi Prisym Id

Step By Step Approach Of Unique Device Identification Udi
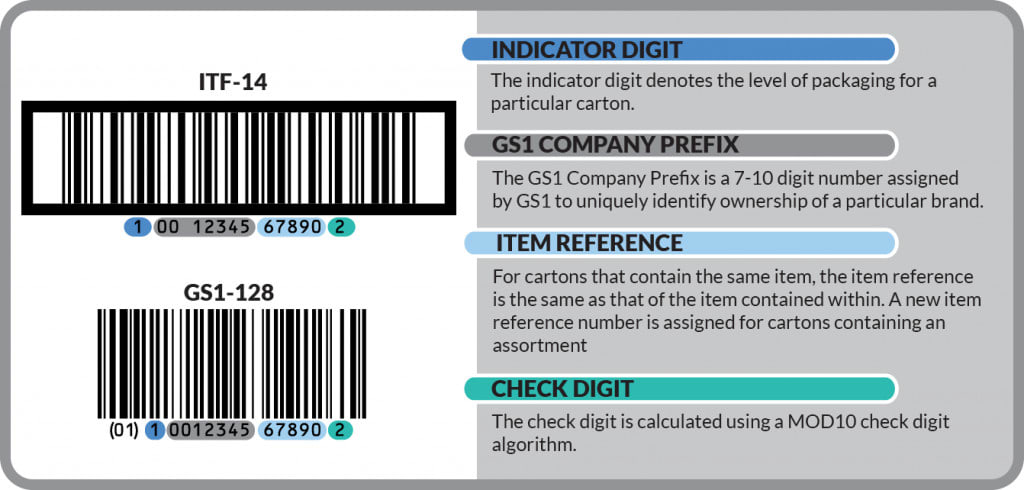
Help For Unique Device Identification Udi Gs1 Gtin Barcode By Sown786
Webinar What Patients Must Know About The Unique Device Identification Udi System

Device Identification Accuracy Random Forest Rdnf Support Vector Download Scientific Diagram

Unique Device Identification Udi What We Must Face

Beckhoff Information System English

Accessgudid The Global Unique Device Identification Database Bluesyemre

Unique Device Identification Udi

Unique Device Identification Olympus Medical Systems

Device Identification Bank Information Security

Beckhoff Information System English

Modbus Step Types Help
Device Id 1 3 2 Download For Android Apk Free

Unique Device Identification Udi Gs1

Faq On Udi Part 1 Facts About The Unique Device Identification Directive Foba Blog

Is It Normal For Zwave Device Identification To Take Hours Bindings Openhab Community

Short Guide To Unique Device Identification By Doranix Doranix Medium

Regulatory Aspects Of Medical Devices In Usa
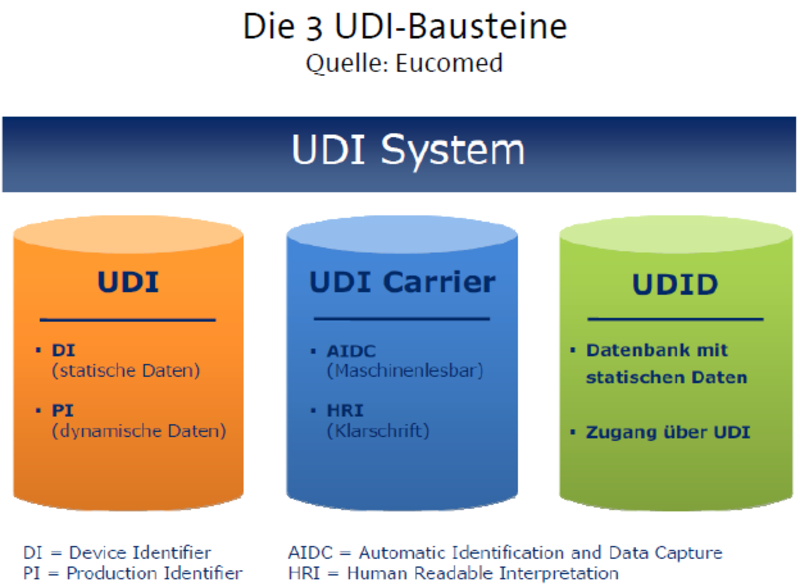
Unique Device Identification Udi

Understanding The Basics Of Unique Device Identification Udi Youtube

Unique Device Identification The New Technology That Could Save Lives Johnson Johnson
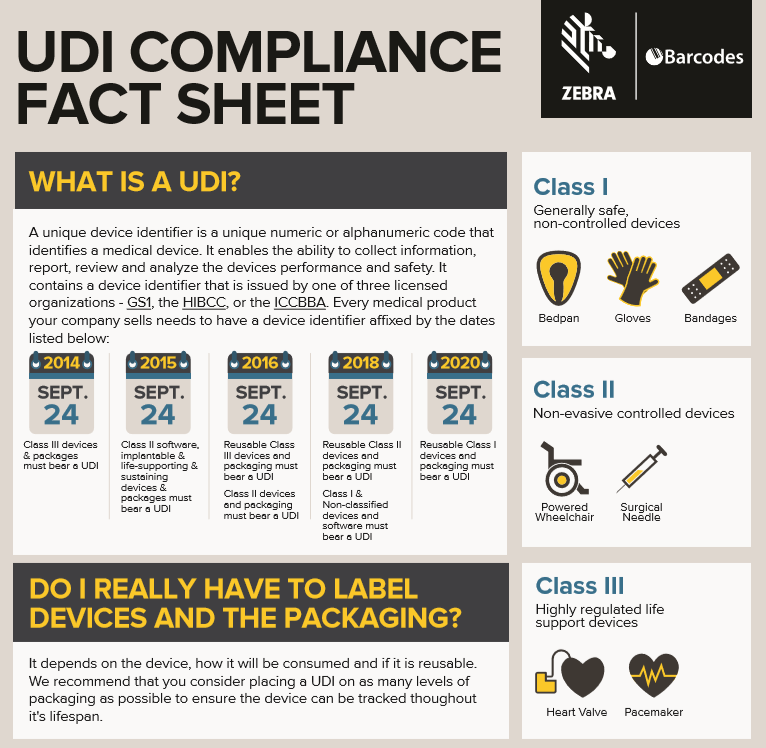
Unique Device Identification Compliance Barcoding Newsbarcoding News

Budget 21 Improving Access To Medicines Unique Device Identification System For The Safety Of Medical Devices Australian Government Department Of Health
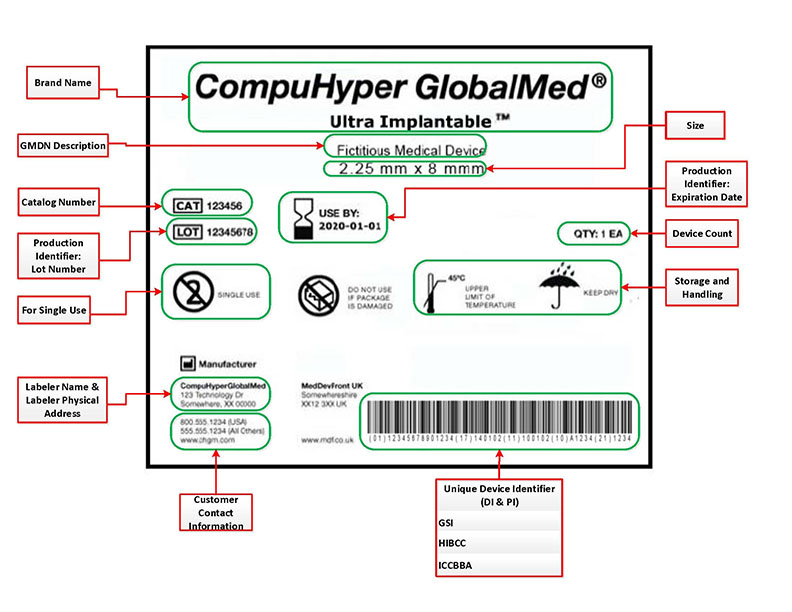
Udi Basics Fda

What Is Cross Device Identification Redeye
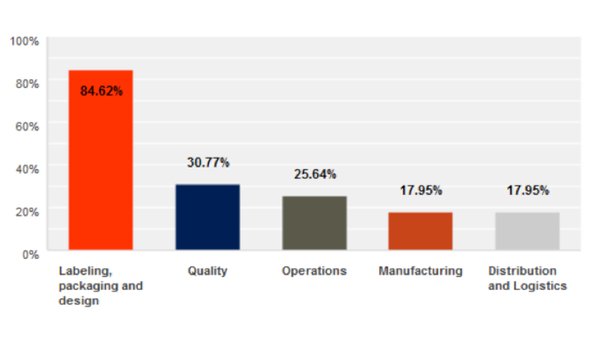
Unique Device Identification How Do You Get It Right Med Tech Innovation Latest News For The Medical Device Industry

Faq On Udi Part 1 Facts About The Unique Device Identification Directive Foba Blog

Unique Device Identification Blog Schmidt

Unique Device Identification Pdf Free Download
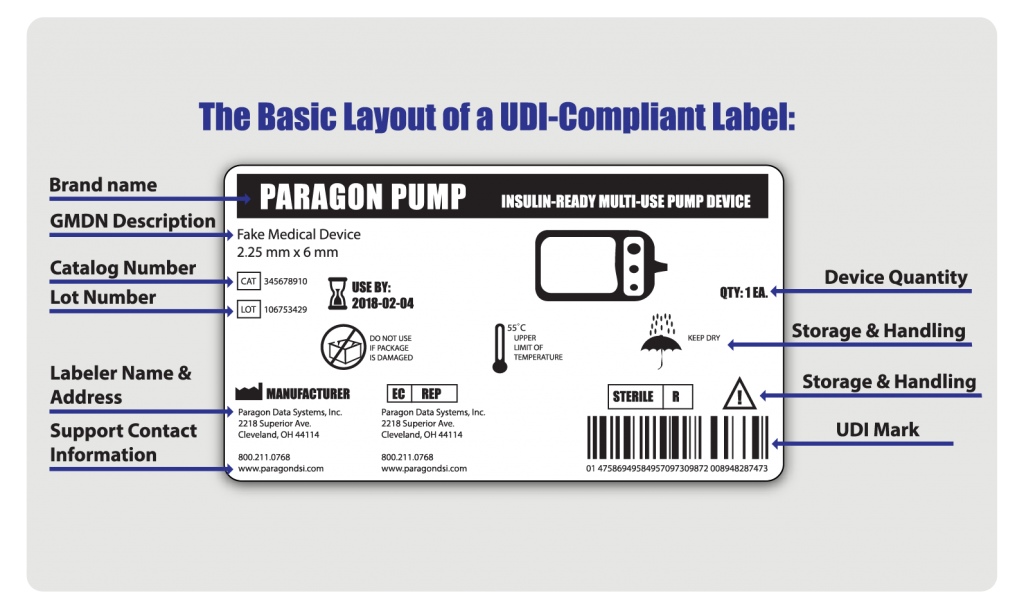
Udi Unique Device Identification For Single And Multiple Uses

Unique Device Identification System Udi System I3cglobal
The Electrical Device Identification Model And Related Questions Download Scientific Diagram

Medical Device Legislation In The European Union Revision Of Current Framework Including Unique Device Identification Udi Regulation Is Coming Soon Kvalito Consulting Group
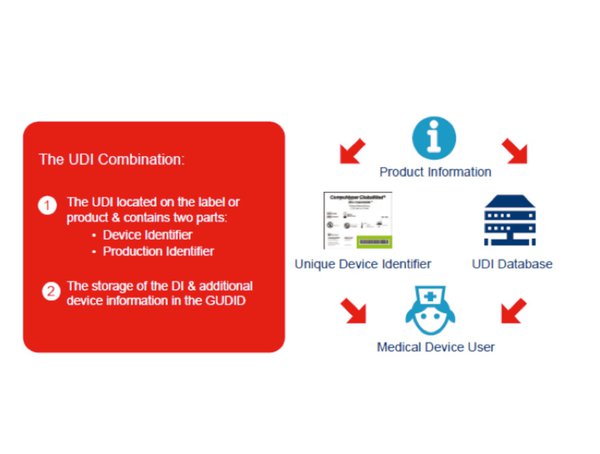
How To Get Unique Device Identification Right Medical Plastics News
1

Udi Beginners Guide Unique Device Identification Eu Mdr And Ivdr
How Can I Read The Device Identification Of The X80 Ethernet Modules By Explicit Message Faqs Schneider Electric Aps Global
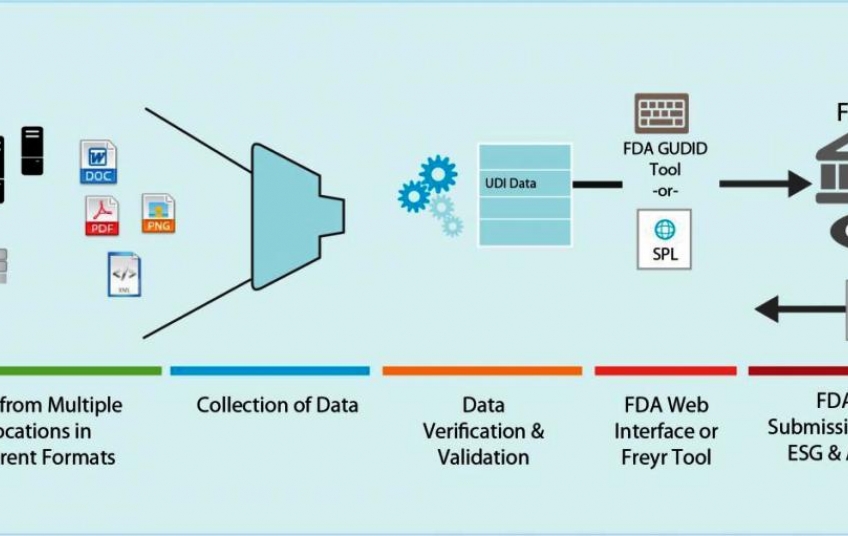
Unique Device Identification
Proposed Approach For Automatic Cross Device Identification Download Scientific Diagram
Kb Fortinet Com Kb Documentlink Do Externalid Fd

Fda Unique Device Identification Udi Overview
1
Comic Cross Device Identification Adexchanger

Mediamath Blog Your Cross Device Identification Questions Answered

Unique Device Identification Make Your Mark Packaging Europe

Iot Device Identification Ppt Powerpoint Presentation Model Show Cpb Presentation Graphics Presentation Powerpoint Example Slide Templates
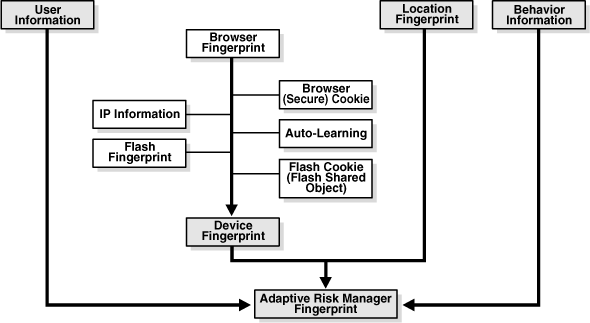
Device Identification

Future Proof Compliance Global Unique Device Identification

Gs1 Unique Device Identification Barcodefaq Com

Udi Unique Device Identification Gs1 Sweden
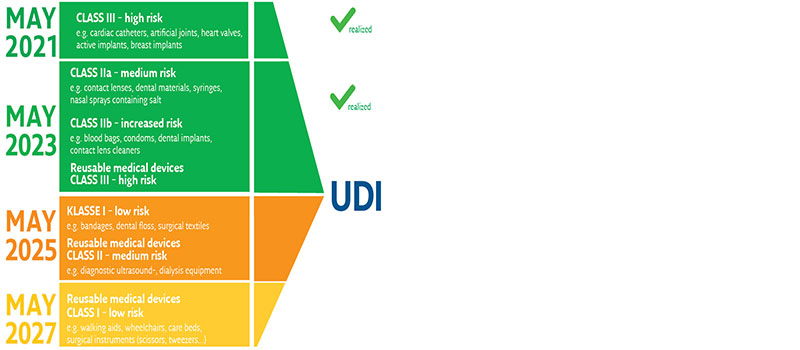
Unique Device Identification Udi Healthcare Gs1

Webinar What Patients Must Know About The Unique Device Identification Udi System

What Is Unique Device Identification Udi Pacific Data Integrators
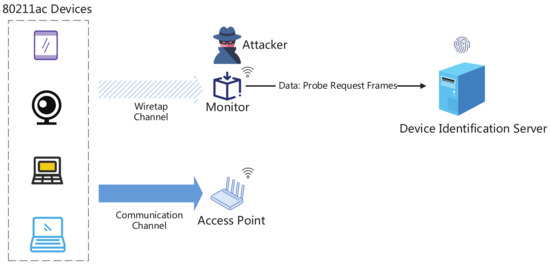
Sensors Free Full Text Probe Request Based Device Identification Attack And Defense

Unique Device Identification

A Focus On Quality The Unique Device Identification System Udi Caq Software Anbieter
Q Tbn And9gcrfhvw29lhl7v64q1uvggxd8ev6imrckofhh2ft8r4 Usqp Cau
Unique Device Identification For Medical Devices Rea Jet

Column Compliance Date Approaching For Fda Unique Device Identifiers Medtech Intelligence
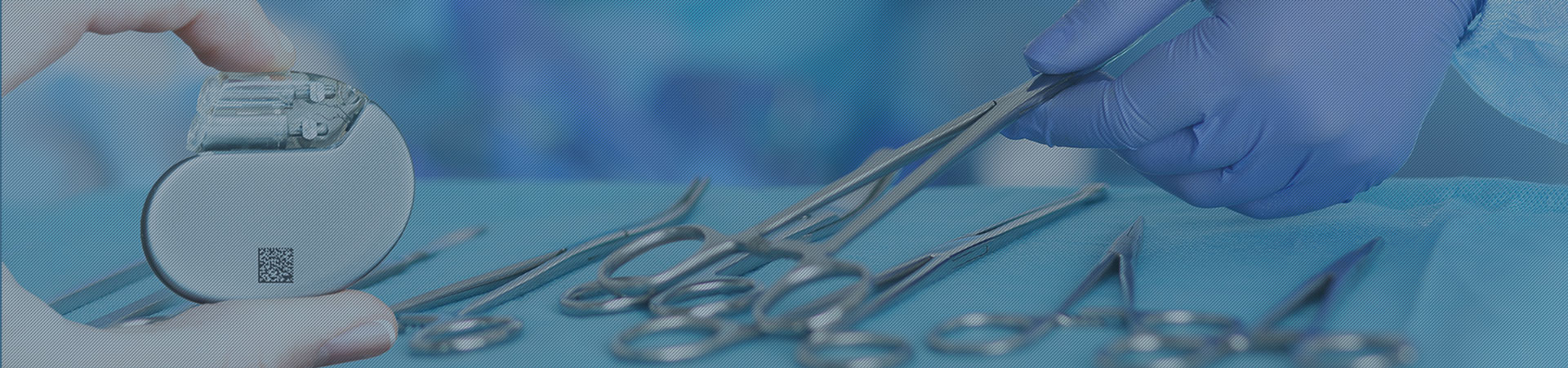
Unique Device Identification For Medical Devices Rea Jet
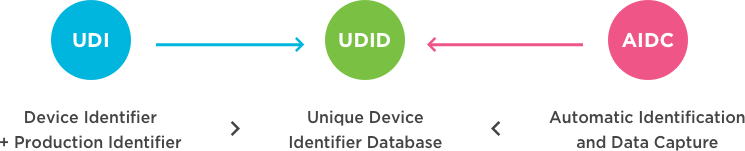
Unique Device Identification Udi Healthcare Gs1
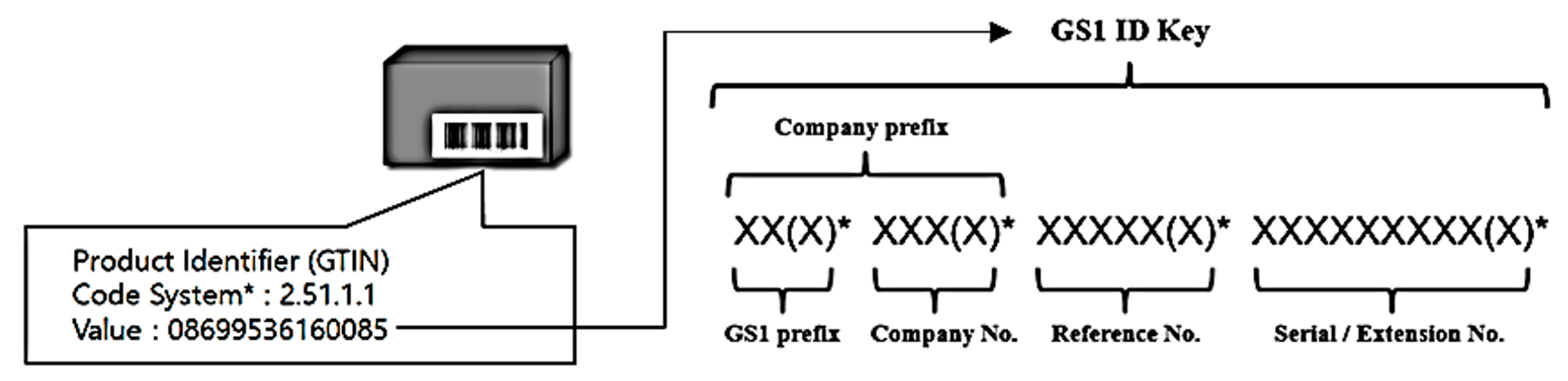
Sensors Free Full Text Device Identification Interoperability In Heterogeneous Iot Platforms

Towards A System Of Unique Device Identification Udi Barbey Avocat Avocat Droit De La Sante

How Europe Can Prepare For Unique Device Identification Udi Medical Product Outsourcing

Fda Final Rule For Unique Device Identification System Policy Medicine

Captiva Spine Upcoming Deadline For Udi Compliance
Http Eur Lex Europa Eu Lexuriserv Lexuriserv Do Uri Oj L 13 099 0017 0024 En Pdf

Help With Device Identification In A Chain Electrical Engineering Stack Exchange
Www Gs1 Org Sites Default Files Docs Healthcare Position Papers Gs1 Udi Guide Final Pdf
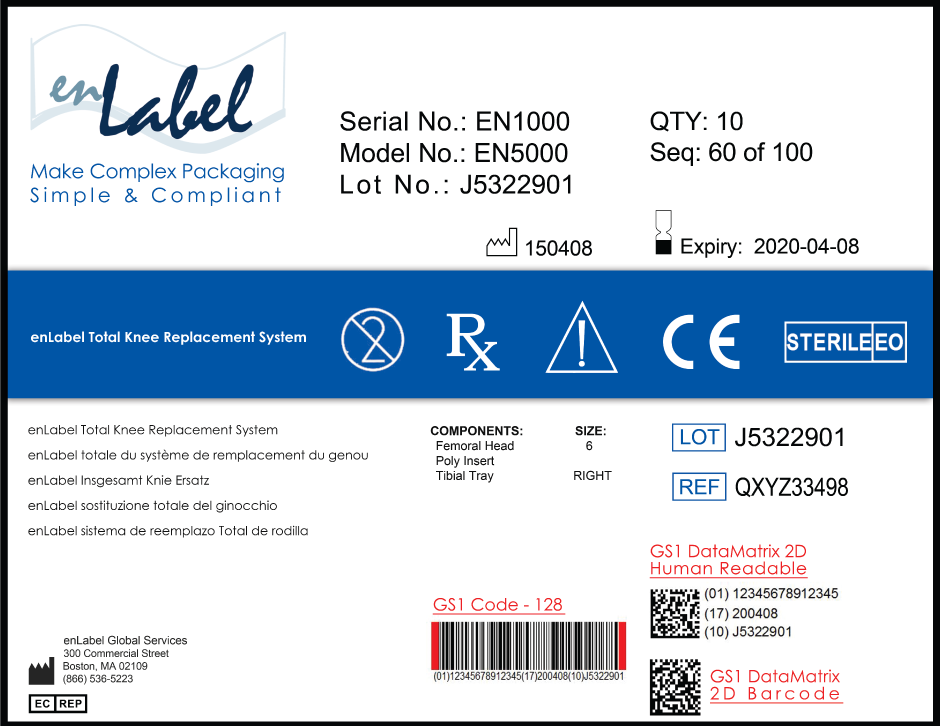
Unique Device Identification Udi Enlabel Global Services

Unique Device Identification Udi Resource Page Medical Device Academy Medical Device Academy

Where Do Windows Product Id And Device Id Values Come From Are They Useful For Licensing Identification How Can They Be Accessed In Code Stack Overflow

Unique Device Identification An Overview Of The Udi System In The Eu

Unique Device Identification Udi For Medical Devices Futurebridge

Unique Device Identification An Overview Of The Udi System In The Eu

Ppt Unique Device Identification And Hl7 Messages Powerpoint Presentation Id

Unique Device Identification Improving Patient Safety Elos Medtech

Does Your Medical Device Have A Unique Device Identification Udi Customs International Trade Law Blog

Udi Beginners Guide Unique Device Identification Eu Mdr And Ivdr
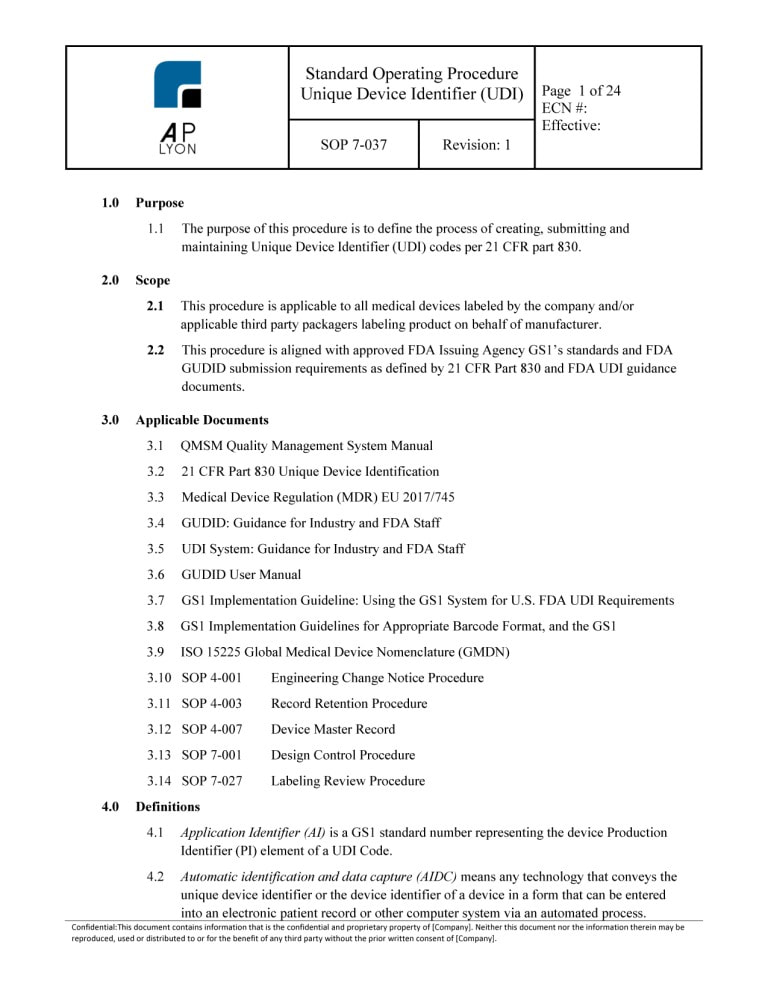
Unique Device Identification Udi Procedure

Unique Device Identification For Medical Devices Pharma Iq

Fda S Unique Device Identifier Successful Implementation
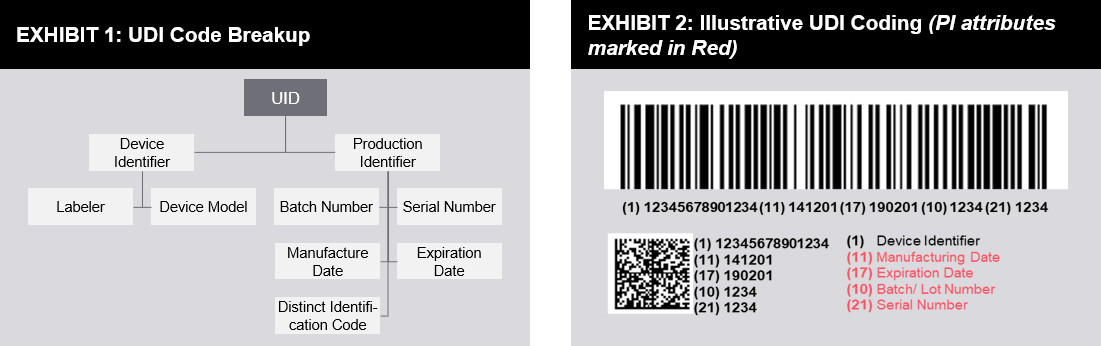
Unique Device Identification Udi For Medical Devices Futurebridge

Gs1 Guide On Unique Device Identification Udi Device Identification Udi Priority By Imposing Legislation For Unique Device Identification For Medical In Vitro Diagnostics

Unique Device Identification Eu Udi Medical Device Ce Marking

What Is The Unique Device Identification Udi Prisym Id

Unique Device Identification Udi What We Must Face

Unique Device Identification And Traceability For Medical Software A Major Challenge For Manufacturers In An Ever Evolving Marketplace Sciencedirect

Traceability Of Medical Devices Vde Medical Devices And Software

What Is Udi Unique Device Identification Tsquality Ch
Device Identification

Live Webinar Udi Unique Device Identification According To New Medical Devices Regulation 05 May Medical Device Trainings



