Authentification Gmp

Carole Achard Service Central D Authentification Universite De

Pdf Current Trends In Supercritical Fluid Chromatography

Shanghai Janteng Trading Co Ltd Quality
Features Matrix Mindmeister Mind Map
Www Jouve Hdi Com Any Run Free Malware Sandbox Online

Bioarmor A Controlled And Acknowledged Quality
Two factor authentication is a subdivision of multi factor authentication simply because it utilizes more than one type of identifier Using more than two types is difficult and can be seen as intrusive by the user, or can be impractical at this moment in time for general commercial use.

Authentification gmp. Self inspection program is conducted in order to monitor the implementation and compliance with current GMP principles and to ensure that the necessary corrective measures are taken 2 Purpose Selfinspection is a way to evaluate the whole operating system from every aspect that might influence the quality of the products It is not only to. This page is only for customers with a previous Maps APIs for Work or Maps API for Business licenseThis page is not applicable to customers with the new Google Maps Platform Premium Plan, which became available in January 16 Client IDs and signatures When using the Google Maps Platform web services with a Google Maps APIs for Work license, two authentication parameters are required. Developer support Does the SSO solution provide APIs and support so you can enable single signon for your custom applications and third.
Title Procedure for Product Identification and Traceability Author https//wwwgmpsopcom Subject The purpose of this SOP is to define the method used for the identification of all contributing materials that could effect product quality used in the manufacture of product, and the final product, to ensure their full traceability. Good Manufacturing Practices (GMP) certification from SGS ensures the integrity of your food manufacturing process as well as your compliance with food safety regulations Assurance of the safety and quality of food is an important consideration for consumers today A GMP is an important enhancement to your food safety management system, which. November 30th, Our office will be closed the following dates December 24th, December 25th, December 31st, January 1st, 21 We apologize for any inconvenience Please call (801) or email authentications@utahgov if you have any questions regarding the closures and Continue Reading.
Published on Monday, January 16, 17 If you want use your Good Manufacturing Practices (GMP) Certificate globally, you may need to have it authenticated and legalized, a process also referred to as Apostille A Good Manufacturing Practices (GMP) Certificate is a Health Canada document that is issued as a part of the quality assurance process that ensures that a drug is consistently produced. 3 These requirements include, for example, certain provisions of the Current Good Manufacturing Practice regulations (21 CFR Part 211), the Quality System regulation (21 CFR Part 0), and the. Dear packagers and users of the Greenbone Source Edition, before updating your sources and packages from GVM/OpenVAS 9 to our latest GVM 10 release, please take a look at the necessary changes for a successful data migration The name and location for the openvasscanner log file has changed too Furthermore the kb_location configuration option of the openvassdconf has been renamed to db_address.
Good manufacturing practices (GMP) are the practices required in order to conform to the guidelines recommended by agencies that control the authorization and licensing of the manufacture and sale of food and beverages, cosmetics, pharmaceutical products, dietary supplements, and medical devices These guidelines provide minimum requirements that a manufacturer must meet to assure that their. Authentication service of IUT Paul Sabatier of Toulouse Auch Castres. Create an account Submission of new papers for GMP 21 is closed Submission of new papers for GMP 21 is closed.
This stepbystep guide is for Australian manufacturers of therapeutic goods (medicines, active pharmaceutical ingredients (APIs) and biologicals, human blood and blood components and haematopoietic progenitor cells) applying for a manufacturing licence for an Australian manufacturing site;. Shree Sai Nath Documentation India Pvt Ltd is India’s first Limited liability firm, established in 1998, which is exclusively engaged in the field of Document Attestation, Authentication, Apostille and Embassy Legalization Shree Sai Nath Documentation India Pvt Ltd is the best service provider in the field of Commercial Documents Attestation, Authentication, Apostille and Embassy Attestation. Title Procedure for Product Identification and Traceability Author https//wwwgmpsopcom Subject The purpose of this SOP is to define the method used for the identification of all contributing materials that could effect product quality used in the manufacture of product, and the final product, to ensure their full traceability.
3 These requirements include, for example, certain provisions of the Current Good Manufacturing Practice regulations (21 CFR Part 211), the Quality System regulation (21 CFR Part 0), and the. This stepbystep guide is for Australian manufacturers of therapeutic goods (medicines, active pharmaceutical ingredients (APIs) and biologicals, human blood and blood components and haematopoietic progenitor cells) applying for a manufacturing licence for an Australian manufacturing site;. SSL authentication configuration for the plugin You can establish a secure connection between IBM® Security Verify Governance plugin and IBM Security Verify Governance server You must configure the plugin and the server to use the Secure Sockets Layer (SSL) authentication.
The process of authentication and legalization of a Good Manufacturing Practices (GMP) Certificate involves three steps Step 1 involves preparing your certificate for submission Step 2 involves submitting your certificate to Global Affairs Canada to be authenticated. When paper records are used, critical GMP actions and decisions are traced to individuals through a handwritten signature This approach applies readily when a computersystem produces a record as a papercopy ‘Electronic signatures’ are the analogous authentication process for fully computerised, ‘paperless’, systems. (5) Digital signature means an electronic signature based upon cryptographic methods of originator authentication, computed by using a set of rules and a set of parameters such that the identity of the signer and the integrity of the data can be verified.
The 3 Main User Authentication Methods for 21 CFR Part 11 Video Course Code ELM It is the accountability of the organizations that implements esignatures to ensure that each electronic signature is unique to one individual and my not be reused or reassigned to anyone else within the organization. Authentication providers can be AD Domains or a claims provider Configuring authentication providers You must configure the adapter with one of the authentication providers that is supported by the Microsoft SharePoint Web Application Authentication providers can be AD Domains or a claims provider. Australian sponsors of therapeutic goods manufactured overseas applying for GMP certification of the.
Authentication in which the signer is asked a number of personal questions, eg “What is (GMP), Good Distribution Practice (GDP), and Good Pharmacovigilance Practice (GVP) Public key infrastructure (PKI) A comprehensive system of roles, policies, and procedures to provide publickey encryption. Please see Authentication Certificate Requirements for more information Check Your Document Status To ask about the status of your documents, please call the Office of Authentications at on Monday through Friday from 800 am to 300 pm. Good Manufacturing Practice (GMP) is a system for ensuring that products are consistently produced and controlled according to quality standards It is designed to minimize the risks involved in any production that cannot be eliminated through testing the final product.
More Information GMP Workstation, PC, Thin Client, Atex IP69k, Touch Screen, Mounting Options RFID/NFC authentication, WLAN/Bluetooth GMP PC Workstation. More Information GMP Workstation, PC, Thin Client, Atex IP69k, Touch Screen, Mounting Options RFID/NFC authentication, WLAN/Bluetooth GMP PC Workstation. Authentication Services Command Line Specific Extensions Compression and Archive Extensions Credit Card Processing Cryptography Extensions Database Extensions Date and Time Related Extensions File System Related Extensions A GMP number representing the bitwise AND comparison.
GOOD MANUFACTURING PRACTICE (GMP) FOR PHARMA COMPANY GMP is part of the QA that ensures that products are produced consistently and regulated by appropriate quality standards in their intended use and as required by authorization for the sale or product for clarification GMP is intended reducing the risk of any pharmaceutical production 12. GMP GMP is a free library for arbitrary precision arithmetic, operating on signed integers, rational numbers, and floatingpoint numbers visualstudio gmp msvc visualstudio Updated Dec 18,. Please see Authentication Certificate Requirements for more information Check Your Document Status To ask about the status of your documents, please call the Office of Authentications at on Monday through Friday from 800 am to 300 pm.
Australian sponsors of therapeutic goods manufactured overseas applying for GMP certification of the. Based authentication will be required (for bank account information, the second set of authentication will still be required) Third Change The status will indicate if the user opted GMP will be storing Taxpayer extract information from CADE2 The system uses shared. Good Manufacturing Practice (GMP) Good Manufacturing Practice (GMP) is a system for ensuring that products are consistently produced and controlled according to quality standards It is designed to minimize the risks involved in any production that cannot be eliminated through testing the final product.
Using GMP¶ The Greenbone Management Protocol (GMP) is the protocol implemented by the Greenbone Vulnerability Manager Daemon – gvmdIt is also used by the Greenbone Security Assistant Daemon to request all of its information from gvmd. Good Manufacturing Practices (GMP) certification from SGS ensures the integrity of your food manufacturing process as well as your compliance with food safety regulations Assurance of the safety and quality of food is an important consideration for consumers today A GMP is an important enhancement to your food safety management system, which. The Enforce identity authentication feature defines the trigger events that prompt a recipient to reauthenticate when interacting with an agreement Opening the agreement is the primary authentication trigger This authentication must be enabled if either of the other triggers is enabled.
This page is only for customers with a previous Maps APIs for Work or Maps API for Business licenseThis page is not applicable to customers with the new Google Maps Platform Premium Plan, which became available in January 16 Client IDs Authentication to the Maps JavaScript API for Google Maps APIs for Work customers requires a unique client ID in combination with URL registration. Authentication Services Command Line Specific Extensions Compression and Archive Extensions Credit Card Processing Cryptography Extensions Database Extensions Date and Time Related Extensions File System Related Extensions A GMP number representing the bitwise AND comparison. Store Information Tealight Innovations Pvt Ltd , (Recognized Startup By Department For Promotion Of Industry & Internal Trade, Ministry Of Commerce & Industry Cert.
Guidelines for Password Management Purpose The purpose of this Guideline is to educate Carnegie Mellon University (“University”) students, faculty and staff on the characteristics of a Strong Password as well as to provide recommendations on how to securely maintain and manage passwords. A GMP certification is given based on a finished GMP assessment of a producer working as per the GMP guidelines The authentication is given that satisfactory documentation on the examination followup is submitted The GMP certification pronounces that the maker agrees to GMP, and the certificate incorporates a reference to the latest date of. Using GMP¶ The Greenbone Management Protocol (GMP) is the protocol implemented by the Greenbone Vulnerability Manager Daemon – gvmdIt is also used by the Greenbone Security Assistant Daemon to request all of its information from gvmd.
Automatic forced authentication for highrisk resources;. Current Good Manufacturing Practices (CGMPs) help to establish the foundation for quality pharmaceuticals through regulatory standards CGMPs, regulations enforced by FDA, provide. GMP service is down This means the manager daemon isn’t reachable Please check if openvasmd/gvmd is running.
Ampoules of this master cell bank (23) should be allocated for quality control confirmation that the cell count and viability of the bank is acceptable and that the bank is free of bacteria, fungi and mycoplasma It is also important at this stage to authenticate the master cell bank by STR profiling, DNA barcoding or SNP analysis Additional tests, such as viral screening, is also required. Good manufacturing practices (GMP) are the practices required in order to conform to the guidelines recommended by agencies that control the authorization and licensing of the manufacture and sale of food and beverages, cosmetics, pharmaceutical products, dietary supplements, and medical devices. This stepbystep guide is for Australian manufacturers of therapeutic goods (medicines, active pharmaceutical ingredients (APIs) and biologicals, human blood and blood components and haematopoietic progenitor cells) applying for a manufacturing licence for an Australian manufacturing site;.
Authentication of botanical species requires the development of sample libraries using validated reference materials A chemometric model must be developed and validated by challenge samples to assure success While visual comparison of spectra is commonly utilized for MIR, this is inadequate for botanical authentication due to. GMP requirements of the local Regulatory Authority and with the specifications in the Marketing Authorisation of the importing country or product specification file for Investigational Medicinal Products The batch processing, packaging and analysis records were reviewed and found to be in compliance with GMP”. November 30th, Our office will be closed the following dates December 24th, December 25th, December 31st, January 1st, 21 We apologize for any inconvenience Please call (801) or email authentications@utahgov if you have any questions regarding the closures and Continue Reading.
Problems to log in?. Authentication of Documents Frequently Asked Question's WalkIn Service Notice TWC Service Animals and their Access to Public Places Hours 800 am 430 pm Monday Friday (call for holiday hours) Processing time is dependent on the number of WalkIn customers. Authentication providers can be AD Domains or a claims provider Configuring authentication providers You must configure the adapter with one of the authentication providers that is supported by the Microsoft SharePoint Web Application Authentication providers can be AD Domains or a claims provider.
Authentication Does the SSO solution provide additional security?. New Zealand Green Health Ltd Green Health Ltd established in 04, locate in Auckland, New Zealand Our products are manufactured in GMP or HACCP registered factories, to Standards accepted in the USA, Australia and Europe. Compliance with Good Manufacturing Practice (“GMP”) is an essential part of the pharmaceutical quality system In particular, through the pharmaceutical quality system it should be ensured that (i) the personnel are adequately trained and there is clear allocation of responsibilities;.
Based authentication will be required (for bank account information, the second set of authentication will still be required) Third Change The status will indicate if the user opted GMP will be storing Taxpayer extract information from CADE2 The system uses shared. Two factor authentication is a subdivision of multi factor authentication simply because it utilizes more than one type of identifier Using more than two types is difficult and can be seen as intrusive by the user, or can be impractical at this moment in time for general commercial use. The 3 Main User Authentication Methods for 21 CFR Part 11 Video Course Code ELM It is the accountability of the organizations that implements esignatures to ensure that each electronic signature is unique to one individual and my not be reused or reassigned to anyone else within the organization.
Australian sponsors of therapeutic goods manufactured overseas applying for GMP certification of the. Authentication gmp_hosting Hosting gmp_storage Cloud Storage gmp_database Realtime Database gmp_crashlytics Crashlytics gmp_performance gmp_durable_links Dynamic Links All products expand_more expand_less Firebase Extensions Install prepackaged, opensource bundles of code to automate common development tasks. Published on Monday, January 16, 17 If you want use your Good Manufacturing Practices (GMP) Certificate globally, you may need to have it authenticated and legalized, a process also referred to as Apostille A Good Manufacturing Practices (GMP) Certificate is a Health Canada document that is issued as a part of the quality assurance process that ensures that a drug is consistently produced.
IUT Département GMP INTRANET. Most GMP functions accept GMP number arguments These are shown in this documentation as GMP objects However, note that PHP 55 and earlier represented GMP numbers as resource s Most of these functions will also accept numeric and string arguments, so long as it is possible to convert the latter to a number. GOOD MANUFACTURING PRACTICE (GMP) FOR PHARMA COMPANY GMP is part of the QA that ensures that products are produced consistently and regulated by appropriate quality standards in their intended use and as required by authorization for the sale or product for clarification GMP is intended reducing the risk of any pharmaceutical production 12.
Microsynth Ag Microsynth Fr

Life Science Laboratory Life Science Siemens Global
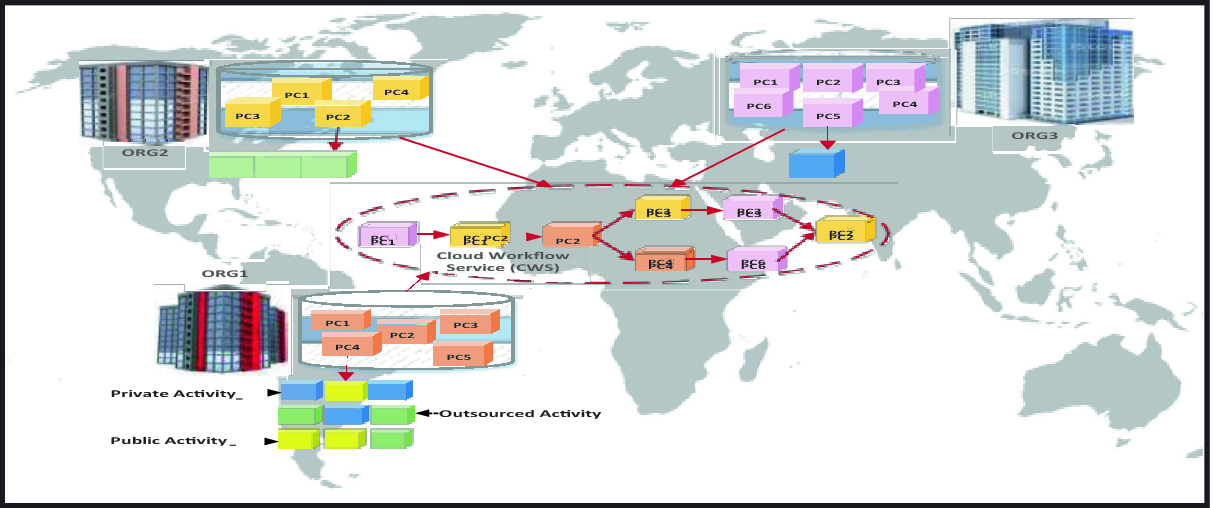
Process Chunks Selection In The Context Of Loose Inter Organizations Cooperation In Cloud Computing Springerlink
Ensign

Authentification Gmp

Roushun Red Pomegranate Niacinamide Hyaluronic Acid For Face Skin Care Serum Buy Niacinamide Serum Hyaluronic Acid Serum Face Serum Product On Alibaba Com

Sarl Gmp Etiquettes Adhesives Berichten Facebook

Woa1 Security Laminates With Interlaminated Transparent Embossed Polymer Hologram Google Patents

Attestation Export Canada Documents For The Middle East Alsc Trade Document Specialists Make It Easy Authentication Legalization Services Canada

Footlocket

Kodementor Kodementor Twitter

Epm May 13 By Epm Magazine Issuu

Understanding Gxp Compliance Gxp Regulations Elpro Cloud

80cv0 Installation Directions
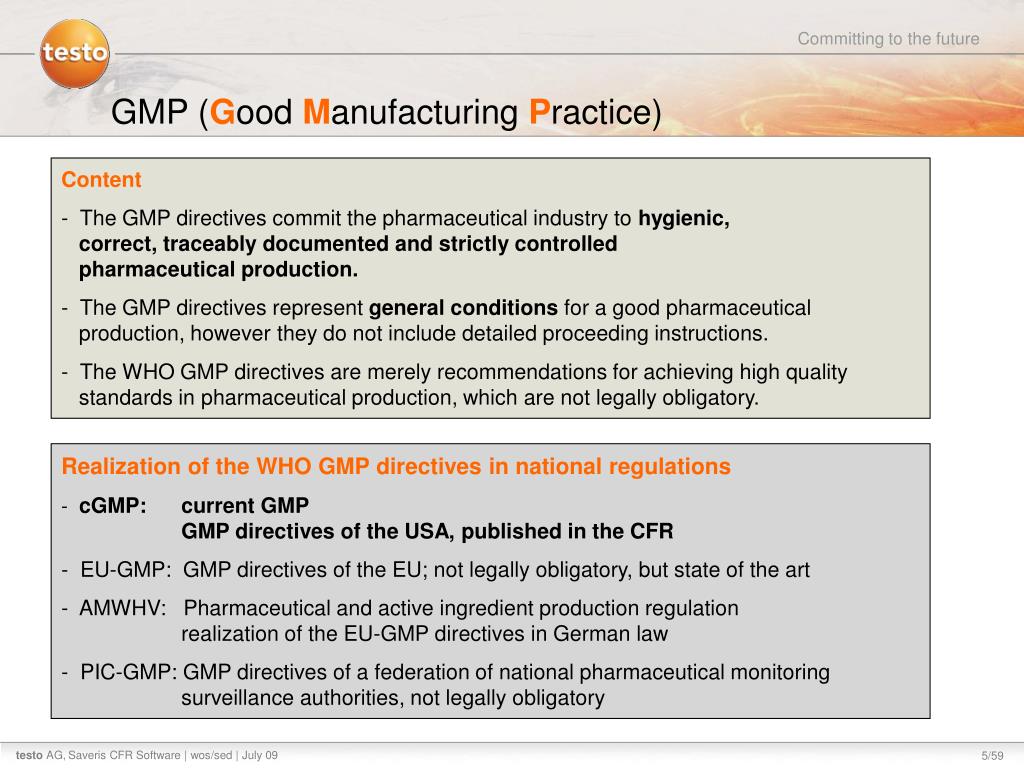
Ppt Market Launch Documentation Testo Saveris 21cfr11 Software July 09 Powerpoint Presentation Id 3340

Sarl Gmp Etiquettes Adhesives Berichten Facebook

Shanghai Janteng Trading Co Ltd Quality

Supply Cocoa Powder Butter And Liquor Flavor Cocoa Powder Drink Buy From Huadong Cocoa Food China Jiangsu European Business Directory European Trade Portal Europe B2b Marketplace

The Fashion Supply Chain Needs A New Tech Makeo

Setup Pydio File Sharing And Sync On Ubuntu 16 04 18 04 With Nginx Mariadb And Php 7 2 Fpm Website For Students

Pharma Business Process Automation Youtube

Pdf Current Status And Major Challenges To The Safety And Efficacy Presented By Chinese Herbal Medicine

Figure 2 From Natural Products Drug Discovery Research In India Status And Appraisal Semantic Scholar

Understanding Gxp Compliance Gxp Regulations Elpro Cloud

Phytochemical Characterisation And In Vitro Anticancer Screeening Of Ethanol Extract Of Chromolaena Odorata Linn

Roushun Snail Serum Face Physician Appearance Of Brightens For More Youthful Skin 50 Ml Buy Snail Extract Serum Whitrning Serum Anti Wrinkle Serum Product On Alibaba Com
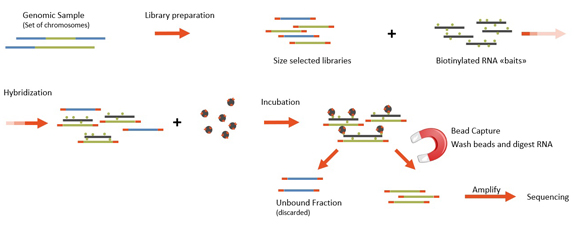
Whole Exome Sequencing At Microsynth Microsynth Fr

Tel 972 Fax 972 Www Coman Org Arenawww Coman Org Quality Management Copyright 01 Dr Alex Coman Ppt Download

Understanding Gxp Compliance Gxp Regulations Elpro Cloud

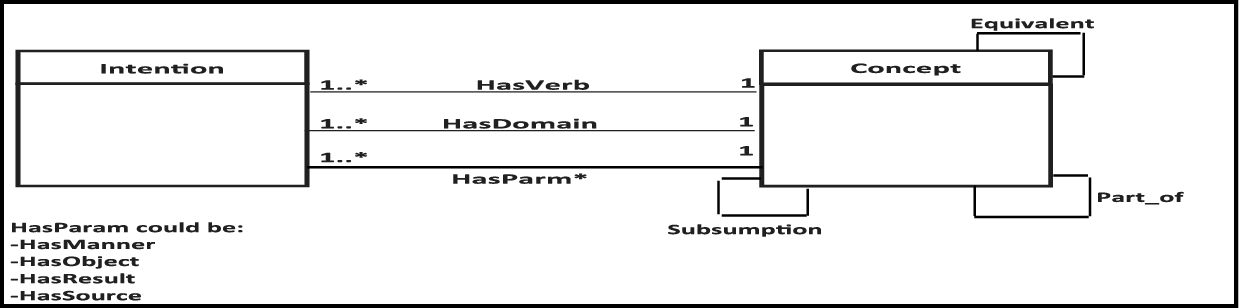
Who Controls The Vocabulary Controls The Knowledge Fbc S

Esteroides Supreme Pharma Mexico
Process Chunks Selection In The Context Of Loose Inter Organizations Cooperation In Cloud Computing Springerlink
Color Contact Lens Tradekorea

Authentification Gmp

Disposable Face Mask 3m 10 Number Of Layers 5 Rs 225 Piece Id

Woa1 Security Laminates With Interlaminated Transparent Embossed Polymer Hologram Google Patents
Easychair Org Publications Download Lpwf

Microsynth Ag Microsynth Fr
Http Nopr Niscair Res In Bitstream 7393 1 Ijeb 48 3 199 7 Pdf
Support Industry Siemens Com Cs Attachments Wcc Options En Us Pdf Download True

Hardware Gmp It Pour Salles Blanches Systec Solutions

Woa1 Security Laminates With Interlaminated Transparent Embossed Polymer Hologram Google Patents

Nickel Oxide And Nickel Salts Copper Oxide And Copper Salts

Universite De Nantes Service Central D Authentification Universite

Woa1 Security Laminates With Interlaminated Transparent Embossed Polymer Hologram Google Patents
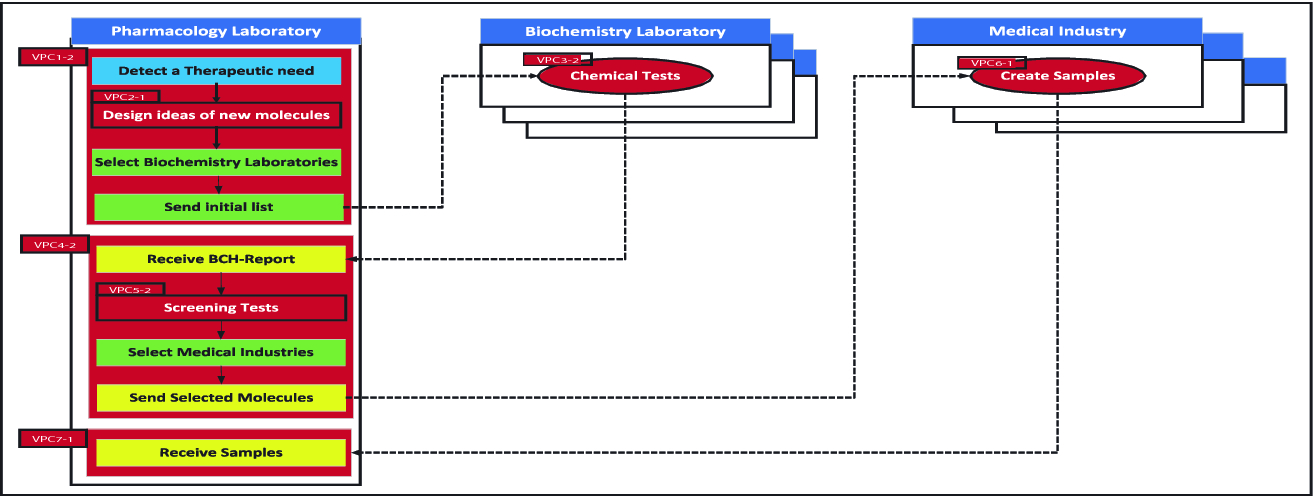
Process Chunks Selection In The Context Of Loose Inter Organizations Cooperation In Cloud Computing Springerlink

The Legal Framework For Electronic Records Storage In France Pierre Saurel Avocat Cabinet Alain Bensoussan Ppt Download

Ensign

Besoin D Un Pc Departement Gmp Genie Mecanique Et Productique De L Iut Universite Polytechnique Hauts De France
Download Cnfcpp Apk Latest Version For Android

21 Cfr Part 11 By Wasan Singhatong Issuu
Understanding Gxp Compliance Gxp Regulations Elpro Cloud

Shanghai Janteng Trading Co Ltd Quality

Probabilites Et Statistique Service Central D Authentification

Sarl Gmp Etiquettes Adhesives Berichten Facebook

Weber Marking Systems Blog Tips En Nieuws Over Etikettering Markering En Coding Pagina 2 Van 3 Weber Marking Systems Blog

Shanghai Janteng Trading Co Ltd Quality

Euromed Communications Blog Euromedcommunications

Roushun Ultra Doux Olive Oil Shampoo And Conditional Vitalizing Plant Normal Hair That Lacks Vitality Buy Olive Oil Shampoo Shampoo And Condition 2 In 1 Shampoo Product On Alibaba Com

Sarl Gmp Etiquettes Adhesives Berichten Facebook

Sarl Gmp Etiquettes Adhesives Berichten Facebook
Www Mt Com Dam Product Organizations Pi Pce White Papers Serialization For Pharmaceuticals Pce Wp Serialization With Te 0615 008 En Web Pdf

Hardware Gmp It Pour Salles Blanches Systec Solutions

Microsynth Ag Microsynth Fr
Http Www Unitels Co Kr R Home M Upload A Download Uid 10 Phpsessid a3c3373c547c7550f35ca0dc5

Edpo News And Events Edpo Epfl
Basic Overview Of Contamination Control In Gmp Facility

China Roushun Argan Carrot Aloe Vera Hair Oil On Global Sources
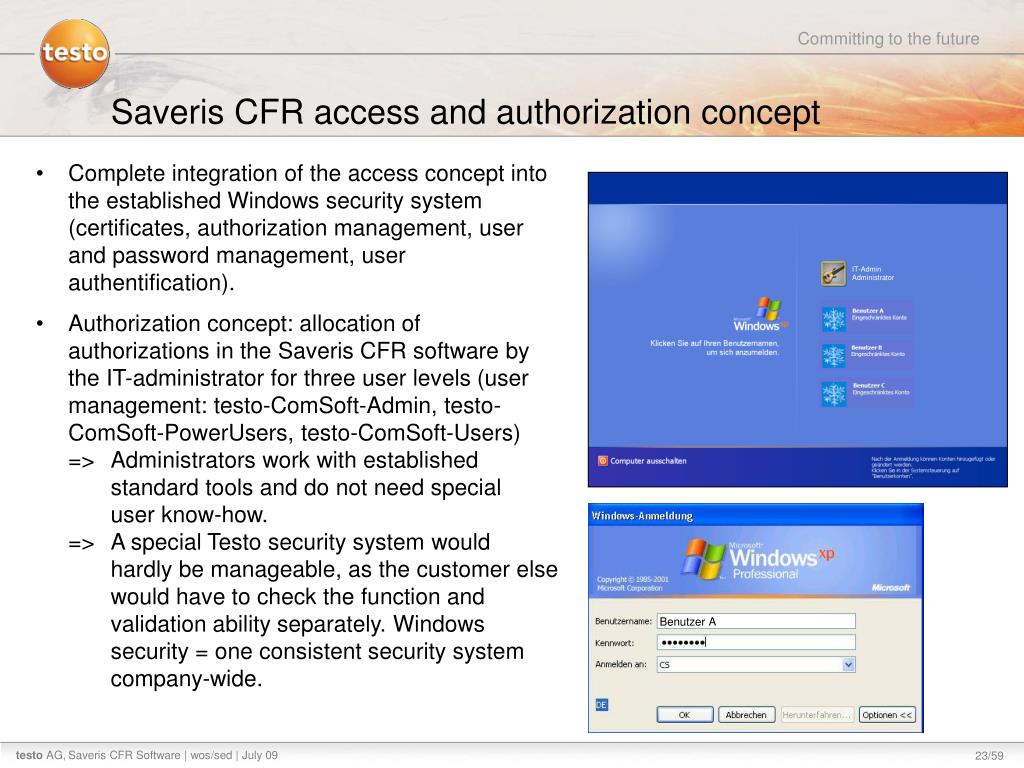
Ppt Market Launch Documentation Testo Saveris 21cfr11 Software July 09 Powerpoint Presentation Id 3340
Http Www Nepad Org Scientificconference Index Php Ct Menu Item 3 Send 8 Presentations Day 2 108 Scomra Presentation Modified Monica
.gif)
Prise En Charge De L Ouverture De Session Par Carte A Puce Pour Les Connexions Vpn D Acces Distant Microsoft Docs

Who Guidelines On Safety Monitoring Of Herbal Medicines In

Characterization And Definition Of Public Performance An Application To Local Government Authorities Cairn Info

Pharma Business Process Automation Youtube

The State Of Medicine Quality In The Mekong Sub Region 4 The Multiple Requirements Of Medicine Quality Institut De Recherche Sur L Asie Du Sud Est Contemporaine
Www Jouve Hdi Com Any Run Free Malware Sandbox Online

Home Kratoshk

Certifications Awards Eoshitech English
Http Www Mm3admin Co Za Documents Docmanager 2d5ed792 878c 4371 9575 81a96bbb26 Pdf

Development History Ensign

Understanding Gxp Compliance Gxp Regulations Elpro Cloud

Pdf Chemical Fingerprinting Of Andrographis Paniculata For Quality Control Studies Using Hplc Technique
Support Industry Siemens Com Cs Attachments Wcc Advanced V13 Sp2 Opt Enus En Us Pdf Download True

Understanding Gxp Compliance Gxp Regulations Elpro Cloud
Understanding Gxp Compliance Gxp Regulations Elpro Cloud
Color Contact Lens Tradekorea
Uvprobe Shimadzu Europa

Cell Line Authentication Microsynth Fr

Life Science Laboratory Life Science Siemens Global

Ensign

Botanical Drugs Challenges And Opportunities Contribution To Linnaeus Memorial Symposium 07 Sciencedirect

Hmis In Oil Gas Applications R Stahl Operating And Monitoring Systems Pdf Catalogs Technical Documentation Brochure

Universite Laval Authentification
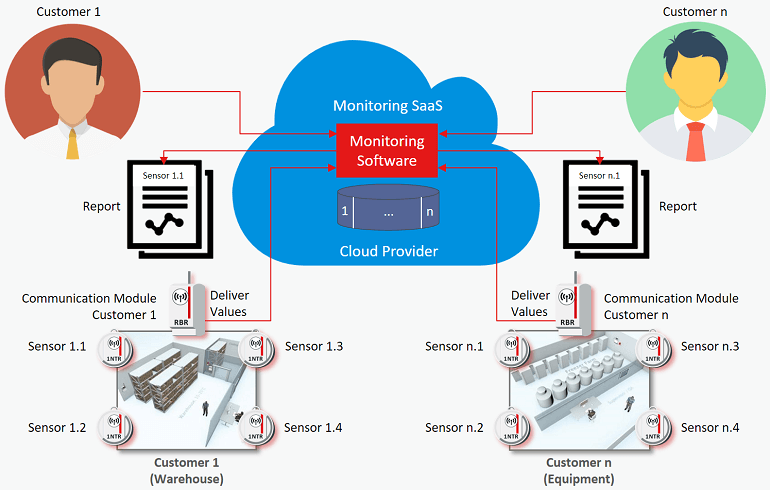
Cloud Based Temperature Monitoring System Elpro Cloud



