Gmp Certification

Taki Mai Taki Mai Kava Shots Haccp And Gmp Certification

Increasing Number Of Eu Gmp Certifications Being Awarded To Canadian Cannabis Companies Cannabis News Box

Gmp Good Manufacturing Practice Certification Agriculture Food

Gmp Certified Essential Oil Company India

Indian Gmp Certification Who Gmp Certification

Our Gmp Certificate Stands For Medicinal Cannabis To The Highest Quality
The training is designed for individuals representing Quality Assurance, Quality Control, Production and Management Individuals of all levels within a pharmaceutical manufacturing organization will benefit by understanding the GMP requirements.

Gmp certification. GMP (Good Manufacturing Practices), created for the producers of food, food contact materials and food additives, is an indicator of compliance to official regulations This worldwideaccepted standard focuses on the proper use of food, food contact materials, as well as food additives,. What is GMP (Good Manufacturing Practices)?. GMP certification is a system which design to ensure the products are consistently produced follow the quality standards Therefore, manufacturers, processors, and packagers are encouraging proactively adopt this system as a result ensure their products are safe and pure.
Good Manufacturing Practices (GMP) certification from SGS ensures the integrity of your food manufacturing process as well as your compliance with food safety regulations Assurance of the safety and quality of food is an important consideration for consumers today. GMP Laboratories of America Awarded NSF cGMP Certification The company was found in compliance with the new federal regulations for dietary supplement manufacturing guidelines under NSF’s vigorous independent GMP auditing program Anaheim, CA— October 14, 09 —GMP Laboratories of America, Inc, has announced certification by NSF. A Good Manufacturing Practices (GMP) certification scheme provides independent verification and certification that the basic manufacturing practices and prerequisites necessary for the implementation of an effective Hazard Analysis Critical Control Point (HACCP) food safety program are being followed.
Certvalue is the top recognized GMP Consultant in Croatia, providing GMP Certification in Croatia, Zagreb, Dubrovnik, Split, Rijeka, Pula, Sibenik with the services of Implementation, Documentation, Audit, gap analysis, training, Registration process at affordable cost to all Good Manufacturing Practice Certification in Croatia industries. The following GMP/cGMP training courses offered by CfPA focus on good manufacturing practices, regulations, and guidelines GMPs InDepth Analysis for Pharmaceutical Life Cycle Management Our NEW GMPs InDepth Analysis for Pharmaceutical Life Cycle Management course will provide an orientation and understanding of the USA FDA’s Current Good Manufacturing Practice for pharmaceutical product. Become a Current Good Manufacturing Practices (cGMP) Certified Professional ™ cGMP Certification Also see GMP FACILITY Certification Program CfPIE has developed a good manufacturing practices training and certification program to meet the educational needs of those responsible for compliance with this complex regulation.
Basics of FDA GMP Training 1 Basic cGMPsA Basic Overview of the US FDA’sRegulations for Regulatory ComplianceCompliance Insight, Inc 2 Basic cGMPs• Remember, QUALITYis the responsibility ofeveryone• Don’t just make theproduct or do your joband leave it up toQuality Assurance fixthe problems 3. Inform your Certification Body From 1 January 21, you can use the GMP notification forms to inform your Certification Body if you are using a gatekeeper protocol. GMP Certification Resource Center Public information and training courses for companies that are looking to be certified in Good Manufacturing Practices Note This site does not provide GMP Certificates Please contact one of our partners for this service.
To achieve EXCiPACT GMP certification, PromoCell was required to demonstrate that its pharmaceutical excipients are manufactured according to the GMP guidelines, as well as ensuring compliance in. GMP Certificate will be issued by a thirdparty organization after inspection of the facility based on a guideline GMP Certificate can be issued by Government organization and Certification Bodies GMP Certificate helps your organization to ensure regulatory compliance while demonstrating your knowledge and commitment to produce safe, quality healthcare products to the public. When Saturday, July 08, 17 900 AM 500 PM (Pacific Time) Where Mandalay Bay, 3950 S Las Vegas Blvd Las Vegas, Nevada 119 United States Phone 800NSFMARK x5600 Email training@nsforg.
The GMP certification scheme defines conditions relating to production facilities as well as for storage, transport, trade and monitoring activities The GMP guideline follows the food chain from primary production through to final consumption, highlighting the key hygiene controls at each stage GMP provides a framework to. What is GMP & why might you need GMP certification?. The GMP certification scheme defines conditions relating to production facilities as well as for storage, transport, trade and monitoring activities The GMP guideline follows the food chain from primary production through to final consumption, highlighting the key hygiene controls at each stage GMP provides a framework to.
To achieve EXCiPACT GMP certification, PromoCell was required to demonstrate that its pharmaceutical excipients are manufactured according to the GMP guidelines, as well as ensuring compliance in. GMP Certification Good Manufacturing Practice was introduced by the Food & Drugs Administration (FDA) under Food Drug Cosmetic Act (Chapter IV for food Chapter V(A)(B. Good Manufacturing Practice (GMP) is a system for ensuring that products are consistently produced and controlled according to quality standards It is designed to minimize the risks involved in any production that cannot be eliminated through testing the final product.
NPA GMP certification and display of the GMP certification seal demonstrates to suppliers, retailers, consumers and the public that products have been manufactured using good manufacturing practices and provide a means of selfassessment and independent evaluation of GMP compliance to the dietary supplement industry. The Good Food Management Practices (also known as GMP's) section contains violations that may cause unsanitary conditions While facilities are expected to address items in this section, these violations are not assessed demerits at this time GMP's are an integral part of the active managerial control within a food establishment Left unchecked, GMP violations may. GMP is typically ensured through the effective use of a quality management system (QMS) Because the FDA requires very specific GMP requirements that differ from those of the EU and other countries, drugs approved or synthesized without US FDA certification cannot be legally sold in the US.
Companies registered for GMP receive instant eligibility for entering products into NSF's product certification program These benefits extend to contract manufacturers registered for GMP through NSF, too When your contract manufacturer is GMP compliant, you decrease the time and cost of NSF testing and product certification. GMP refers to the Good Manufacturing Practice regulations promulgated by the US Food and Drug Administration under the authority of the Federal Food, Drug, and Cosmetic Act (See Chapter IV for food, and Chapter V, Subchapters A, B, C, D, and E for drugs and devices). Good Manufacturing Practices (GMP) is a system that ensures manufacturing products, such as food, cosmetics, and pharmaceutical goods, are consistently produced and controlled according to set quality standards.
GMP Certification Good Manufacturing Practice was introduced by the Food & Drugs Administration (FDA) under Food Drug Cosmetic Act (Chapter IV for food Chapter V(A)(B. Certvalue is the top recognized GMP Consultant in Croatia, providing GMP Certification in Croatia, Zagreb, Dubrovnik, Split, Rijeka, Pula, Sibenik with the services of Implementation, Documentation, Audit, gap analysis, training, Registration process at affordable cost to all Good Manufacturing Practice Certification in Croatia industries. Sample certificate of completion Course Completion Certificate When answers to the quiz questions for all 12 modules have been submitted, the student will receive a “Certificate of Course Completion” by email to demonstrate that they have completed this GMP internet training course.
GMP stands for Good Manufacturing Practices, a certification that is awarded by the FDA to manufacturers who are in compliance with the highest manufacturing standards GMP will sometimes appear as cGMP for Current Good Manufacturing Practices, but it more or less denotes the same meaning. About cGMP Certification The cGMP Certification is needed by manufacturing companies to manufacture and sell food and drug related products The GMP, or Good Manufacturing Practices, are the guidelines that decide whether or not a company is allowed to sell their product to the general public. This certification demonstrates understanding and implementation of the principles underlying the laws, regulations, directives, standards, guidance, compendia for manufacturing, testing, and holding for finished pharmaceuticals and raw materials.
GMP stands for Good Manufacturing Practices, a certification that is awarded by the FDA to manufacturers who are in compliance with the highest manufacturing standards GMP will sometimes appear as cGMP for Current Good Manufacturing Practices, but it more or less denotes the same meaning. EU GMP certification is the highest recognition available by companies in the pharmaceutical space European Union Good Manufacturing Practices details the production, handling, storage and packaging of cannabis The Canadian cannabis industry as a whole has been trending toward global expansion. Why GMPCertification is Important The pharmaceutical quality of medicines and supplements is regulated very carefully, as it affects the lives of nearly every American Manufacturing facilities must be designed and monitored properly, and the products they create must follow identity, strength, quality, and purity guidelines.
A Good Manufacturing Practices (GMP) Certificate can be issued by companies, which are able to document adherence to Good Manufacturing Practices, as outlined by the US FDA, State Health Departments, or by another set of standards Most countries prefer that GMP certificates be issued from a trade association, such as ACMA. GMP certification ensures that products are produced following the quality standard norms GMP certification deals with issues like documentation, record keeping, personnel qualifications, sanitation, cleanliness, equipment verification, sanitation, complaint handling, and process validation GMP requirements are not peculiar in any sense. GMP (Good Manufacturing Practices), created for the producers of food, food contact materials and food additives, is an indicator of compliance to official regulations This worldwideaccepted standard focuses on the proper use of food, food contact materials, as well as food additives,.
Good Manufacturing Practices (GMP) is a system that ensures manufacturing products, such as food, cosmetics, and pharmaceutical goods, are consistently produced and controlled according to set quality standards. The Certified Pharmaceutical GMP Professional understands the good manufacturing practices (GMP) as regulated and guided by national and international agencies for the pharmaceutical industry. GMP certification is usually only requested if it is not possible to obtain GMP clearance via the Mutual Recognition Agreement (MRA) or Compliance Verification (CV) pathways, for example due to lack of evidence The TGA reserves the right to undertake an audit of an overseas manufacturing site, irrespective of any other evidence supplied.
The following GMP/cGMP training courses offered by CfPA focus on good manufacturing practices, regulations, and guidelines GMPs InDepth Analysis for Pharmaceutical Life Cycle Management Our NEW GMPs InDepth Analysis for Pharmaceutical Life Cycle Management course will provide an orientation and understanding of the USA FDA’s Current Good. To achieve EXCiPACT GMP certification, PromoCell was required to demonstrate that its pharmaceutical excipients are manufactured according to the GMP guidelines, as well as ensuring compliance in. Good Manufacturing Practice Standard Good Manufacturing Practice Standard Good Manufacturing Practice (GMP) is a vital component of Quality Assurance to help ensure that therapeutic products and Chinese Proprietary Medicines (CPM) are consistently produced with the quality standards appropriate for their intended use.
GMP certification audit After our GMP auditors have completed the GMP certification audit and find that your company has demonstrated satisfactory compliance with the required GMP standard, we will issue the certificate in the name of your company as registered with the Accounting and Corporate Regulatory Authority The scope of certification. In the kratom industry, one of the most important standards, by far, is AKA GMP (Good Manufacturing Practice) Certification This article discusses the exacting standards required for this prestigious certification, why it’s important for users’ health and safety, and why you should only shop with AKA GMP certified vendors like Kratom Spot. GMP Certificates for online training course completion Elearning training courses are a budgetwise option for good manufacturing practice (GMP) training for orientation or updates, when combined with other onsite training as required for your products.
Pharmaceutical GMP Audits and SelfInspections Learn how to perform your best audit and become a Certified GMP Pharmaceutical Quality Systems Lead Auditor Investigational Medicinal Products QP Module Led by former IMP inspectors and QPs, this interactive course adds value to QPs, auditors and clinical trial supply staff. The acronym stands for Good Manufacturing Practices, and to be GMP certified means that the manufacturer has demonstrated a strong regulatory commitment and compliance to international GMP standards 5 Key Considerations for Choosing a Pharmaceutical Contract Manufacturing Facility. The GMP Certificate is granted after a facility has been audited and has been found to have demonstrated that they are complying with the standards required to fulfill the requirements of the GMP certification The certificate is usually valid for a period of three years and will be issued in the name of the registered company.
What is GMP & why might you need GMP certification?. The Training and Qualifications Compliance Manager will interface with regulatory stakeholders (egSummary The Training and Qualifications Compliance Manager is responsible for managing Allegiant learning and training record systems with an emphasis on maintaining system integrity to ensure. CGMP refers to the Current Good Manufacturing Practice regulations enforced by the FDA.
The GMP training seminar and course provides a complete overview of the industry requirements as specified by the FDA Who Should Attend This GMP Training This threeday Good Manufacturing Practices training is designed for those who work in a cGMP environment, from beginners to advanced professionals. GMP Labs™ is a NSF GMP for Sport Registered Facility and USDA Organic certified manufacturer that operates out of two facilities, totaling over 100,000 sq ft, both located in the Anaheim Center for Advanced Technology in California. With over GMP FSA certified companies, it is the largest module within the GMP certification scheme GMP FSA contains standards for all links in the chain Certified companies are audited by independent bodies, accepted by GMP International, the socalled Certification bodies.

Gmp Certification Good Quality Must Be Built In During By Tnv Seo Medium

Gmp Certification Good Manufacturing Practice Supplement Factory Uk

Nsf Gmp Registration Program Definition What Is Nsf Gmp Registration Program Find Nsf Gmp Registration Program Companies On Thomasnet Com Certification Search

Good Manufacturing Practice Course Gmp Certified Program Cfpie

Gmp Logo Good Manufacturing Practice Logo Certification Good Manufacturing Practice Text Monochrome Png Pngegg

The United Laboratories Non Sterile Clavulanate Potassium Have Obtained Gmp Certificate In China Federal Pharmaceutical News Dynamics Http Lbzy 0756u Cn En

Gmp Good Manufacturing Practice Certification And Consultancy Service In Rohini Delhi International Accurate Certification Id

Good Manufectering Practice Gmp Certificate In Hub Town Ahmedabad Epic Consultants Id

Good Manufacturing Practice International Certifications

Theracell Accreditations

Services Gmp Certification In India From Delhi Delhi India By Prime Vision Consultancy Services Id

We Passed Nsf Audit And Got Nsf Gmp Certification Successfully Yuwei Biotechnology

The Green Organic Dutchman Provides Update On Eu Gmp Certification

Gmp Certification In Delhi Good Manufactu Ultimate Certification Services Pvt Ltd

Certificates West Coast Pharmaceuticals

Rentschler Fill Solutions Received Unlimited Gmp Certification

National Meditek Who Good Manufacturing Practice Gmp Certification National Meditek

Gmp Certification Asmi International Pvt Ltd

Good Manufacturing Practices Certification Gmp Benefits Approval Guidelines Certification Youtube

Download

Sgs Certifies Dows Stade Site With Excipact Gmp Standard

Gmp Certificate Tm Media Titan Biotech Ltd

What S Better Than Gmp Certification

Gmp Certification Documents Manual Requirements

Everyone Wins The Benefits Of An Excipient Gmp Certification Programme Epm Magazine

Gmp International Gmp International

Gmp Certification Services In Mumbai India

Gmp Certification Uk Accredited
Q Tbn And9gctyret8ks3glygsbmvd33mveefuprxrdkm1ndv7jmlnhn9jny W Usqp Cau

Eu Gmp Approved Pharma Company In India Eu Gmp Certified Beta Lactam Antibiotics Medicef Pharma

South Korea Gmp Certification For Medical Devices Asia Actual Llc

Gmp Certification Polycrystalline It

Who Gmp Certification Service In Goregaon E Mumbai Cdg Inspection Ltd

Gmp Certification Hero Nature

Good Manufacturing Practice Course Gmp Certified Program Cfpie

Why Selecting A Gmp Certified Supplement Manufacturer Matters

Gmp Certification

Magicalflavour Passed Gmp Certification
.jpg)
Know All About Gmp Certification
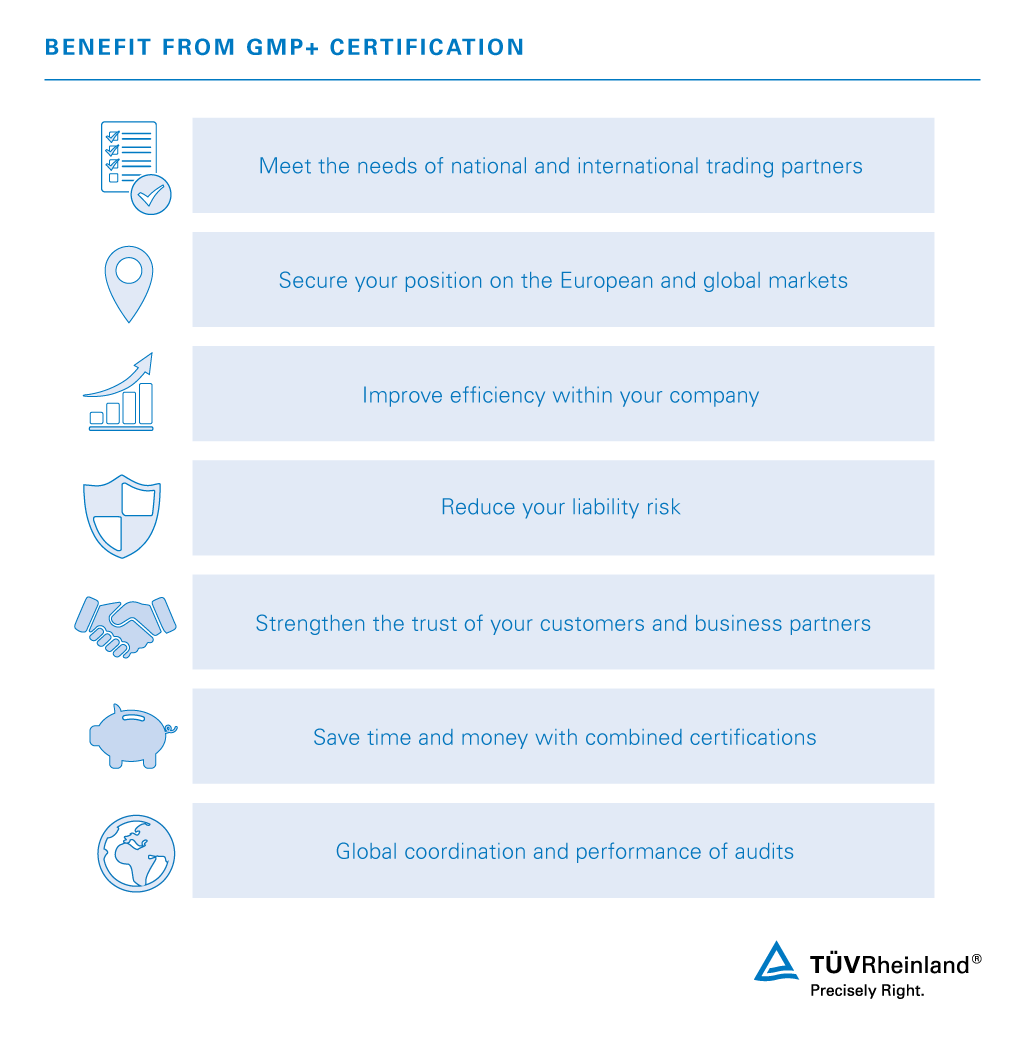
Certification According To Gmp Ru Tuv Rheinland

Good Manufacturing Practices Certification In Kuwait Britishwings Associates Iso Certification

Gmp Certified Manufacturing Quality Assurance Safe For Consumption

Hermes Pharma Obtains Prestigious Gmp Certification From Russian Authorities

Gmp Certification Services Who Gmp Certification Services Service Provider From New Delhi

Europe Chitin

Procedures And Requirements Of Gmp Certification Of Pharmaceutical Drugs In The Philippines Media

Gmp Certification Good Manufacturing Practice Supplement Factory Uk

Who Gmp Certification Service ज एमप सर ट फ क शन सर व स ज एमप सर ट फ क शन स व ए In West Vinod Nagar Delhi Max Quality Assurance System Id

Kfda Gmp Certification

Gmp Good Manufacturing Practice Certified Stamp White Background Vector Stock Vector C Calin Hanga Gmail Com

Who Gives Gmp Certification Smpnutra Com Premier Manufacturing

Who Gives Gmp Certification Smpnutra Com Premier Manufacturing

Gmp Certification Is The Best Way To Measure The Quality Of Goods Iso Standard Certification Accredited By Ukas

Gmp Good Manufacturing Practice Certified Round Stamp On White Royalty Free Cliparts Vectors And Stock Illustration Image

Gmp And Gdp Certification Programme Eca Academy

W R Grace Co Site In Baltimore Maryland Usa Receives Excipact Gmp Certification As Pharmaceutical Excipient Supplier Pharma Excipients

Certification According To Gmp Ru Tuv Rheinland

Gmp Images Stock Photos Vectors Shutterstock

Clr Obtains Effci Gmp Certification Cossma

Gmp Certification Services In Kandivali East Mumbai Id

Iso 07 C Gmp Certification For Cosmetics Good Manufacturing Practices C Gmp In Dhule International Certifications Id

Gmp Certified Vaadi Herbals India

Gmp Certification Services In India Gmp Certification In Uttar Pradesh

Tuv Sud Codex Gmp Certificate Certificates Hsiehs Biotech

Aphria One Receives Eu Gmp Certification To Export Cannabis
Q Tbn And9gctyret8ks3glygsbmvd33mveefuprxrdkm1ndv7jmlnhn9jny W Usqp Cau

New Ipec Guide Gmp Certification Scheme And Certification Body Qualification Guide For Pharmaceutical Excipients Articles

Good Manufacturing Practices Part 117 Online Course Institute For Food Safety At Cornell University

Gmp Certificate Attestation Zambia Embassy Gmp Legalization

Gmp Good Manufacturing Practice Stock Vector Illustration Of Label Certified

Hermes Pharma Obtains Prestigious Gmp Certification From Russian Authorities

Good Manufacturing Practice Quality Certification Png 570x570px Good Manufacturing Practice Badge Best Practice Brand Certification Download

Gmp Certification Hubei Masteam Bio Tech Co Ltd

Uqsr
.jpg)
Gmp Certification Taiwantrade Com

Services Gmp Certification From Gujarat India By Zee Consultancy Service Id

Gmp Certification Service Who Gmp Certification Service Service Provider From Rajkot

Gmp Archives Eu Gmp Consulting And Robotic Solution In Pharmaceutical Industry

What Is Good Manufacturing Practice Certificate And What Is A Benefits Of Gmp Certification By Rishabh Parihar Issuu
Samyang Corporation Customer Center Samyang Corp News Samyang Corporation Received Iran Gmp Certification For Pharmaceutical
Q Tbn And9gct8nmxblzv8mlrenvlia Uy7ye49ujjyrpu4xy6lohwgkf8p8gt Usqp Cau

Clever Leaves A Leading Multi National Cannabis Operator

Gmp Certification Services In Vijay Nagar Indore Id

Impact Of Gmp Certification For Health Supplements By Urs India Issuu

1 Gmp Supplement Manufacturer Ssmfg

Gmp Certification Dga Certification

Gmp Inglot Cosmetics Makeup Skincare Nails Accessories

Why Gmp Certification Matters Paragon Labs Usa

Gmp Certification Documents Manual Requirements

Who Gmp Certification Services For Manufacturing Id

Good Manufacturing Practice Gmp Certification

Gmp Certification Xetley S A

Olimp Labs 1 In 19 We Received Good Manufacturing Practice Gmp Certification 2 Gmp Covers All Aspects Of Production From The Starting Materials Equipment To Packaging Procedures Are Essential For Each

Allan International Holdings Ltd

Gmp Gdp Training Certificate Proof Of Your Qualification Eca Academy
Q Tbn And9gcsy5fwb8e Ve5wikebgyuyjp8hdheonnn7xk6j6ourvkhd1wwl6 Usqp Cau

Current Good Manufacturing Practices Cgmp Audits Ul

Certification According To Gmp Ru Tuv Rheinland



